
Battery’s Solid Electrolyte Interphase: Shocking Plot Twist in Battery Science
November 22nd, 2023 | Reading Time 3 mins
A revolutionary discovery challenges beliefs on performance degradation. Filmy deposits aren’t the primary cause; recent findings reveal the true battery breakdown source
Gear up for a battery blockbuster as recent research flips the script on battery performance blame. Long accused filmy residues and mossy lithium deposits are debunked villains, unveiling a surprising twist in the electric secrets concealed within batteries. In a three-decade quest, scientists cracked the code of the Solid Electrolyte Interphase (SEI), revealing it as a dynamic semiconductor rather than a dull insulator. This revelation transforms the SEI from an enigma to a dazzling science spectacle, rewriting the narrative of battery behavior. Who would have thought batteries could hold such a plot twist?
SEI’s Adaptable Interplay with Battery Components
The complex structure isn’t a solo performer; it adjusts and adapts based on the materials in the cathode and anode, orchestrating a harmonious interplay with the electrolyte composition. Imagine the SEI’s composition, structure, and thickness engaged in a dynamic interplay, responding to the nuances of the electrode surfaces.
Hold on, because the SEI layer isn’t just for show; it’s a backstage maestro influencing the core of battery operations. From shaping electrode material reactivity to directing oxidation/reduction reactions, it serves as the invisible force guiding critical performance aspects like cycle life and safety measures.
To unlock the secrets of more enduring batteries, the key lies in the delicate art of adjusting the liquid electrolyte. Increased electrical conductivity becomes the focal point, creating a denser SEI that navigates the delicate balance between conductivity and the complexity of the SEI.
SEI’s Conundrum in Harsh Battery Environments
In the challenging conditions of extreme battery operation—think high temperatures (>60 °C), rapid charge rates, and prolonged electrochemical cycles—the Solid Electrolyte Interphase (SEI) faces a dilemma. It’s a toss-up between the SEI layer thickening or losing its protective prowess, triggering performance decline through various aging mechanisms.
Despite its paper-thin appearance, the SEI layer is a maestro of selective conductivity. Picture this: during discharge, lithium ions effortlessly traverse this thin layer, while it orchestrates the controlled movement of electrons powering the battery. The continuous evolution of the SEI involves changes in composition, marked by an uptick in inorganic components like Lithium Fluoride (LiF) and Lithium Carbonate (Li2CO3) and a dip in the concentration of organic compounds such as Lithium Alkyl Carbonates.
The SEI’s life story begins with its formation during the initial charging cycle, ideally ensuring stability throughout the battery’s lifespan. However, as batteries age, they often reveal the unwelcome presence of solid lithium buildup on negative electrodes. It’s a tale of resilience and challenges in the microscopic world of battery science.
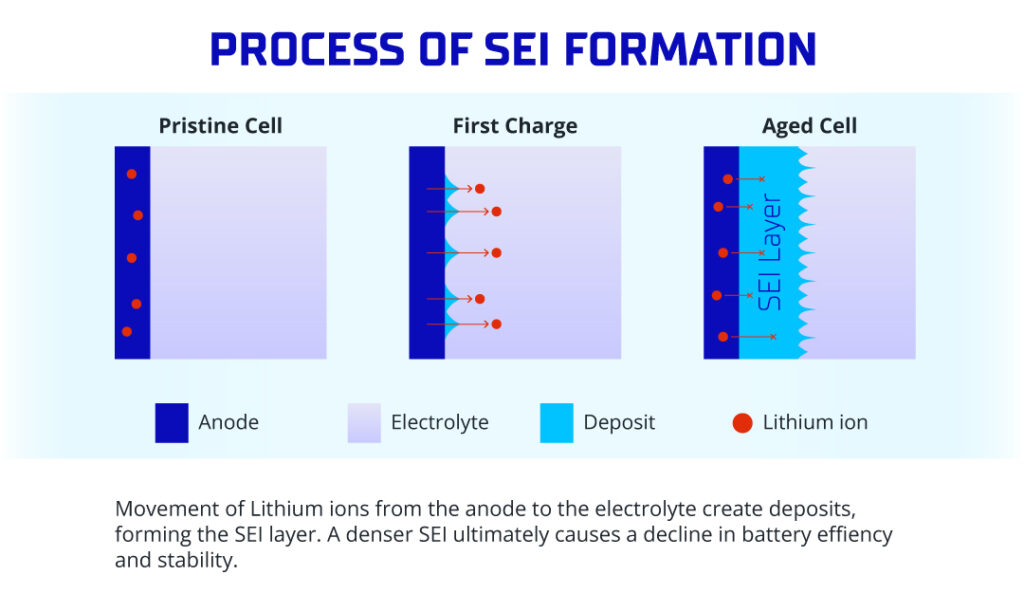
Electron Leakage in the SEI Formation Process
Tackling measurement challenges head-on, researchers pioneered inventive techniques. By marrying transmission electron microscopy with nanoscale manipulation, they embarked on a journey to measure electrical conduction across the Solid Electrolyte Interphase (SEI), uncovering a consistent electron leakage as voltage levels increased.
The SEI formation unfolds in two intricate steps, although the precise mechanism remains a topic of ongoing debate. First, the graphite electrode undergoes polarization, triggering reductive decomposition in the organic electrolyte, birthing new compounds. In the second act, these fresh compounds come together, precipitating to form the SEI until every nook and cranny of the graphite surface finds its coverage. A fascinating twist emerged as researchers discovered that organic components housing carbon within the SEI were culprits of electron leakage. The potential remedy? Minimizing these components emerged as a promising solution to stretch out the battery’s lifespan. It’s a scientific saga of challenges met with innovative solutions in the ever-evolving world of battery research.
SEI’s Role in Revolutionizing Battery Technology
SEI’s surprising shift from insulator to semiconductor challenges established beliefs, shaking the foundations of conventional wisdom. Recognizing the unintended consequence of solid lithium buildup unveils pathways to enhance battery lifespan.
Through tweaks to the physical and electrochemical properties of the liquid electrolyte, we hold the keys to crafting batteries that endure longer and perform at greater heights. This revelation emphasizes the pivotal role of the SEI in dictating battery performance and ensuring safety.
But hold onto your hats because this breakthrough is not a finale; it’s a kickoff. Grasping the intricacies of the SEI not only paves the way for prolonged battery life but also addresses more extensive challenges in energy storage. As the journey of research persists, we find ourselves teetering on the edge of a groundbreaking era in energy storage, promising revolutionary changes across our devices, transportation, and power consumption. The future is buzzing with possibilities!





